Blog
Why Sulfur in Marine Fuel Demands Accurate Analysis
August 6, 2025
Sulfur in marine fuels contributes to acid rain and particulate emissions, posing serious environmental and human health risks. To combat this, MARPOL Annex VI strictly regulates sulfur content in marine fuels such as bunker oil, pushing shipping operators and regulatory bodies toward precise sulfur quantification.
Enter Horiba’s SLFA Series
Horiba’s SLFA analyzers leverage Energy-Dispersive X-Ray Fluorescence (EDXRF) to detect sulfur in petroleum-based samples with high speed and low detection limits. With a dedicated X-ray tube optimized for sulfur excitation and a fully enclosed, safe design, these analyzers eliminate the need for gas utilities or radiation certification.
How It Works
- Pour 4-10 mL of marine oil into a sample cup, seal it with Mylar film and locking ring.
- The instrument launches with pre-calibrated curves – just press Start.
- Within seconds, live data appears. The software then automatically selects optimal calibration (low or high sulfur), computes the final sulfur concentration, and outputs statistical indicators. Results and data can be exported via USB.
Horiba’s default calibration uses certified reference materials spanning 0‑4% sulfur, minimizing matrix effects automatically and ensuring accuracy across fuel compositions.
Why Choose SLFA for Marine Fuel QC?
- Speed and simplicity: Onboard or lab analysis in just minutes.
- Regulatory compliance: ASTM D4294 / ISO 8754 / JIS‑equivalent methods supported.
- Portable option: SLFA‑60’s small footprint and no gas requirement make it ideal for on‑ship testing.
- High throughput: SLFA‑6800 supports autosampling for QC laboratories.
- Minimal operator training: Ready-to-run software, curve auto‑selection, USB export.
Key models:
- SLFA‑60: Compact and portable – Ideal for onboard testing (vessels, tankers).
- SLFA‑6800: Laboratory-grade unit featuring an 8-position autosampler for enhanced throughput and productivity in QC labs.
Performance Metrics
|
Model |
Range |
Repeatability |
Detection Limit |
|
SLFA‑60 |
0–10% S |
≤ 15 ppm |
~20 ppm |
|
SLFA‑6800 |
0–10% S |
≤ 5 ppm |
~5 ppm |
These analyzers deliver consistent, high-precision results across various marine fuels – even with differing elemental matrices (Ca, Zn, V, Ni) – thanks to sophisticated internal filtering and calibration correction algorithms.
Real-World Comparison with Contract Labs
Testing with real ferry and bunker fuel samples showed close alignment between SLFA readings and contract lab results. For example, samples with ~0.7% sulfur showed agreement within ±0.02–0.03%, demonstrating robustness across sulfur levels ranging from 0.02% up to >3%.
Read the full application note here
Maintaining Quality Across the Battery Lifecycle
July 30, 2025
Modern society relies heavily on portable electrical power – supplied primarily by batteries. From smartphones and laptops to tablets and electric vehicles, none would function without a reliable power source. Since Alessandro Volta developed the first battery in the 18th century, countless battery types have emerged, varying in chemical composition and size, and serving applications ranging from flashlights to large-scale energy storage for power grids.
Regardless of a battery’s size, purpose, or whether it’s a primary (non-rechargeable) or secondary (rechargeable) system, elemental analysis of individual components (particularly for carbon, sulfur, oxygen, and nitrogen) is often essential. Instruments like the ELEMENTRAC CS-i and ONH-p2 analyzers enable accurate and reliable measurement of C/S and O/N across a broad concentration range.
ELEMENTRAC analyzers assess the entire sample using combustion or inert gas fusion, ensuring comprehensive, consistent results from trace ppm levels to high percentages.
Why the Full Element Picture Matters
Battery materials are chemically complex – and impurities like sulfur or nitrogen can degrade performance, stability, and cycle life. Even small oxygen residues can catalyze unwanted reactions in electrodes.
In battery production labs, Eltra’s CS‑i is frequently used to quantify sulfur in lead paste – delivering precise values within minutes. Despite sample heterogeneity, repeatability often lands around 0.5% RSD, highlighting its robustness even under challenging conditions.
With ONH‑p, labs can characterize nitrogen and oxygen levels in ceramic separators, silicon-based anodes, and solid electrolytes, empowering designers with critical insights into impurity-driven behavior
Interested in implementing C/S or O/N/H analysis in your lab?
ATS Scientific and ELTRA can help you configure the right solution based on your workflow, sample type, and throughput needs.
The ELEMENTRAC ONH-p2 analyzer
- Uses fusion furnace (>3000 °C) for deep gas extraction
- Detects O, N, H from the ppm range to high concentration levels
- Sample prep: typically 50-1000 mg in nickel capsule or special basket depending on sample nature
- Fast measurement: 2-4 minutes per run
The ELEMENTRAC CS-i Analyzer
- Measures carbon and sulfur from 0.6 ppm to 64 % in one gram of sample
- Ideal for powders or solids (e.g. lead paste, cathode materials)
- Analysis takes just 40–60 seconds per sample
- Typical sample weights: 250–500 mg, with accelerator agents to prevent swirling and incomplete combustion
Click here to read the full application report with real life samples.
What's in in your batteries?
July 22, 2025

Microwave-assisted sample preparation of α-spodumene: A simple procedure for analysis of a complex sample.
Raw materials in the Li-ion industry are often complex matrices, which requires aggressive sample preparation conditions. The following study is focused on the development of a reliable protocol for the chemical analysis of a raw material, the α-spodumene, using microwave sample preparation. The aluminosilicate structure of α-spodumene has to be destroyed through the sample preparation in order to bring all the target elements in solutions and perform their analysis.
Taking into account the technological relevance of Li-ion batteries for electric vehicles, the authors describe how a simple and practical procedure without using concentrated HF was developed for chemical analysis of α-spodumene.
Unlocking Battery Potential: Why Ball Milling Is Powering the Next Generation of Energy Storage
July 15, 2025
.jpg)
As the world races toward electrification, lithium-ion batteries are being pushed to new limits – higher capacity, faster charging, and longer lifespans. Behind the breakthroughs in chemistry and cell design lies a crucial enabler: particle engineering.
At the heart of that process is a trusted workhorse – the ball mill.
What Is Ball Milling – and Why Does It Matter?
Ball milling is a mechanical process used to reduce the particle size of solid materials. In the battery industry, it's much more than just grinding – it’s an essential tool for:
- · Activating surfaces of powders
- · Homogenizing slurries
- · Enabling solid-state synthesis
- · And even coating or modifying particles
By carefully controlling particle size, surface area, and morphology, ball mills help optimize electrode behavior, electrolyte interactions, and even cycling stability – all of which impact real-world battery performance.
How Do Ball Mills Support the Battery Value Chain?
Ball mills play a role at every stage of battery innovation – from material development to recycling:
1. Active Material Synthesis
High-energy ball mills like the RETSCH Emax allow researchers to synthesize novel cathode materials via mechanochemical methods. This dry, solvent-free approach is not only eco-friendly but also enables materials with unique properties that are hard to achieve using traditional wet chemistry.
2. Particle Size Optimization
Cathodes like NMC and anodes like graphite or silicon must be milled to precise size ranges. Too coarse, and you risk poor energy density. Too fine, and you lose conductivity or encounter agglomeration. Ball mills offer tight control to hit the “sweet spot.”
3. Slurry Homogenization
Before being coated onto current collectors, battery slurries must be well-mixed. Planetary ball mills ensure uniform particle dispersion, leading to smooth, consistent coatings with fewer defects.
4. Recycling and Recovery
Spent batteries are crushed and milled to recover valuable metals. Cryogenic ball milling, for instance, allows for low-temperature size reduction of plastic-encased battery components, improving yield and purity.
From Cryo to High-Energy: Solutions for Every Stage

- · CryoMill: Ideal for polymers and heat-sensitive materials
- · MM 500 nano: Ultra-fine grinding at nanoscale
- · Emax: High-energy milling for rapid mechanochemistry
- · PM series: Planetary mills for routine powder preparation and mixing
With accessories like temperature-controlled jars, automated grinding protocols, and contamination-resistant materials (e.g., zirconia or tungsten carbide), these mills are tailored for demanding battery R&D environments.
Milling Toward Better Batteries
From the outside, a ball mill might look like a simple machine – but in the battery world, it’s a precision tool shaping the materials that define energy storage innovation.
For more details and study case data, download the full Retsch whitepaper:
Unlocking the Potential: The Role of Ball Mills in Battery Technology (PDF)
How Science Keeps Your Battery from Falling Apart: The Role of Slurry Stability in Lithium-Ion Cells
July 9, 2025
 Behind every powerful smartphone, electric vehicle, or energy storage unit is a lithium-ion battery – and behind every lithium-ion battery is something much less visible but equally important: a stable slurry.
Behind every powerful smartphone, electric vehicle, or energy storage unit is a lithium-ion battery – and behind every lithium-ion battery is something much less visible but equally important: a stable slurry.
What’s a Battery Slurry?
In battery manufacturing, slurries are mixtures of active materials, binders, solvents, and additives used to coat the electrodes inside the battery. These coatings need to be uniform, smooth, and durable – because even tiny inconsistencies in the slurry can lead to big problems like:
- · Uneven electrode surfaces
- · Poor adhesion
- · Reduced battery life
- · Increased failure risk
So what makes a good slurry? The answer lies in dispersion stability – and that’s where chemistry meets cutting-edge measurement tools.
The Hansen Approach: Matching “Like with Like”
The Hansen Solubility Parameter (HSP) approach is a clever way to predict whether materials will mix well and stay dispersed. HSP breaks down a solvent’s behavior into three forces:
- · Dispersion (δD) – like Van der Waals forces
- · Polarity (δP) – based on molecular polarity
- · Hydrogen bonding (δH) – self-explanatory!
By plotting these parameters in 3D space, formulators can visualize whether a binder and solvent are “compatible.” Think of it like a compatibility map – if two materials sit close together in this space, they’re likely to form a stable mixture.
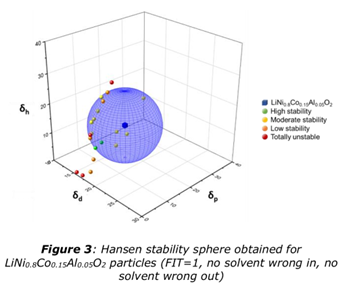
This method helps researchers choose the right combinations and reduce costly trial-and-error during formulation. But even the best predictions still need real-world confirmation.
Measuring Stability with Static Multiple Light Scattering (SMLS)
While HSP gives you the theoretical “match,” real-world confirmation is critical. That’s where Static Multiple Light Scattering (SMLS) comes in.
Unlike traditional visual observation or endpoint analysis, SMLS captures the full kinetics of instability—such as sedimentation, creaming, or aggregation—as they happen. The TURBISCAN sends a light beam through the sample and measures how it scatters as particles migrate or separate. This allows for:
· .bmp) Quantify how stable a slurry really is
Quantify how stable a slurry really is
· Detect early signs of particle agglomeration
· Optimize the timing for coating or processing
The result is a much faster, more sensitive understanding of how your slurry behaves over time – without needing dilution, centrifugation, or prolonged shelf-life testing. By applying the Hansen approach and verifying it with SMLS measurements, labs can drastically reduce trial-and-error– and get to better battery performance.
Why It Matters for the Battery Industry
The battery industry is in a constant battle with the "battery trilemma" - the challenge of simultaneously achieving high energy density, fast charging, and long cycle life. Improving one often means compromising another. Manufacturers are under pressure to push all three boundaries at once, while keeping production scalable and cost-effective.
This makes every step of the battery manufacturing process critical – especially slurry preparation. Poor dispersion or unstable mixtures can lead to uneven coatings, wasted materials, and inconsistent performance. But by applying Hansen Solubility Parameter (HSP) analysis alongside Static Multiple Light Scattering (SMLS), formulators gain better control over slurry behavior. The result: less trial and error, reduced waste, and more reliable, high-performing electrodes that help move the needle on all three fronts of the battery trilemma.
Want to learn more? Read the full technical application note here:
Optimize Lithium-Ion Battery Slurries Using the Hansen Approach

Call Today 1-800-661-6700